Foundational Model for Immunotherapy Outcomes, Vessel Segmentation in X-Ray Angiography, ChemIQ 🚀
Health Intelligence (HINT)
2025-05-19
🚀
COMPASS: Foundation Model for Predicting Immunotherapy Outcomes
Researchers at Harvard Medical School and Roche introduced COMPASS, a concept bottleneck foundation model designed to predict immunotherapy responses across cancers using tumor transcriptomic data. Unlike traditional models that struggle with generalizability and interpretability, COMPASS captures shared immune response principles, enabling accurate predictions even in unseen cancer types and therapies.
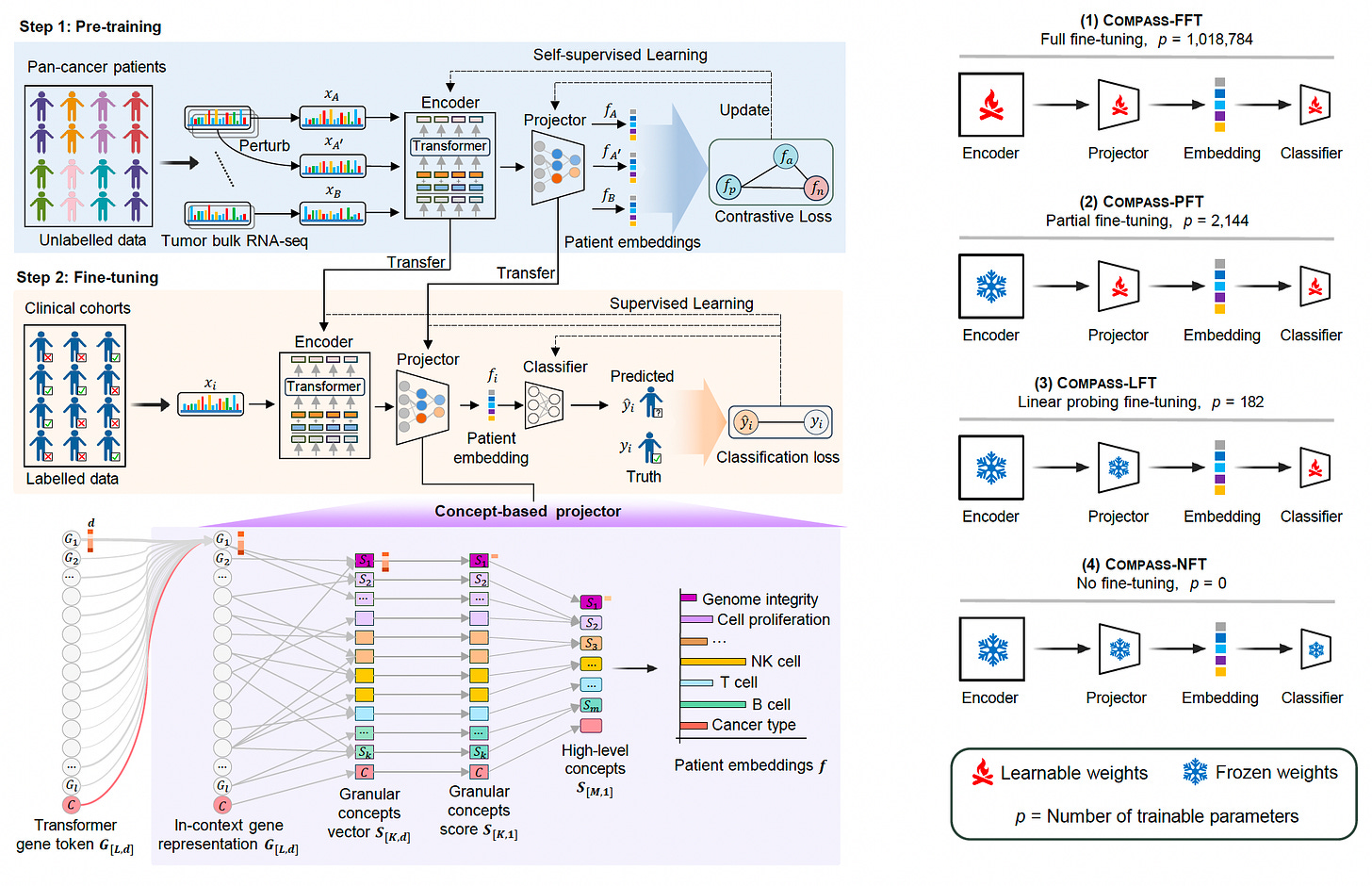
Trained on 10,184 tumors from 33 cancers, COMPASS significantly outperforms 22 existing models, achieving a 15.7% improvement in precision-recall metrics and demonstrating strong survival prediction power (HR = 4.7, p < 0.0001).
Utilized a biologically grounded architecture with 44 immune concepts, modeling immune cell states, tumor-microenvironment interactions, and signaling pathways to deliver interpretable predictions.
Achieved cross-cancer and cross-therapy generalization, accurately predicting treatment responses in cancers like stomach adenocarcinoma (83.7%) and therapies such as anti-CTLA4 (76.1%), even with minimal fine-tuning.
Generated personalized response maps that linked gene expression to immune mechanisms, identifying resistance programs such as TGF-β signaling, endothelial exclusion, CD4+ T cell dysfunction, and B cell deficiency.
Demonstrated clinical utility by supporting patient stratification and indication selection in early-phase trials, providing a mechanistic foundation for trial design and biomarker discovery.
COMPASS offers a powerful, interpretable solution for immunotherapy response prediction, advancing precision oncology and supporting informed clinical decision-making.
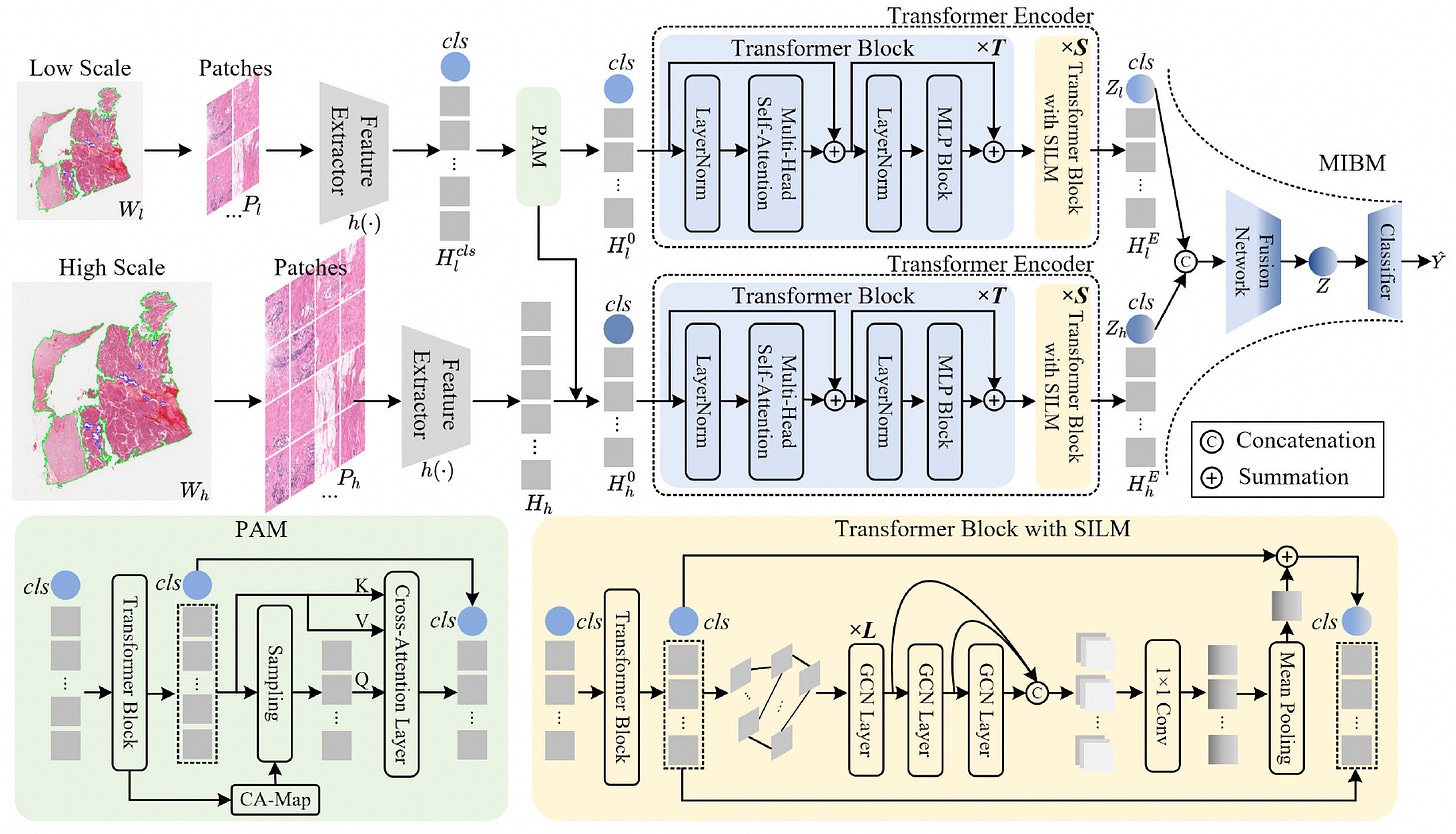
A new study introduces MG-Trans, a multi-scale graph Transformer model designed to improve whole slide image (WSI) classification in digital pathology. By addressing the limitations of traditional multiple instance learning (MIL) methods, MG-Trans enhances both accuracy and interpretability while reducing computational overhead.
Tested on three cancer subtyping and seven gene mutation detection datasets, MG-Trans consistently outperforms state-of-the-art methods, achieving significant improvements in F1 score and area under the curve (AUC) metrics.
Employed a Patch Anchoring Module to select a small, fixed number of highly informative patches from large WSIs, reducing redundancy and focusing on diagnostically relevant regions.
Introduced a Dynamic Structure Information Learning Module that explicitly modeled local tissue structures using dynamically constructed tissue graphs, improving discrimination of subtle cancer subtypes.
Applied a Multi-Scale Information Bottleneck Module to effectively fuse multi-scale patch features, producing a compact and robust bag-level representation that enhances generalization.
Validated superior performance with extensive ablation studies and interpretability analyses, demonstrating accurate tumor localization and critical region identification that align with pathologist annotations.
MG-Trans sets a new benchmark for efficient and interpretable WSI classification, paving the way for more reliable AI-assisted cancer diagnostics.
TVS-Net: Temporal Vessel Segmentation in X-Ray Angiography
Researchers from the University of Oxford developed TVS-Net, a temporal vessel segmentation network that leverages multi-frame X-ray angiography sequences for accurate coronary vessel segmentation. Unlike conventional single-frame models, TVS-Net incorporates temporal dynamics to address motion artifacts and contrast inconsistencies inherent in invasive coronary angiography (ICA).
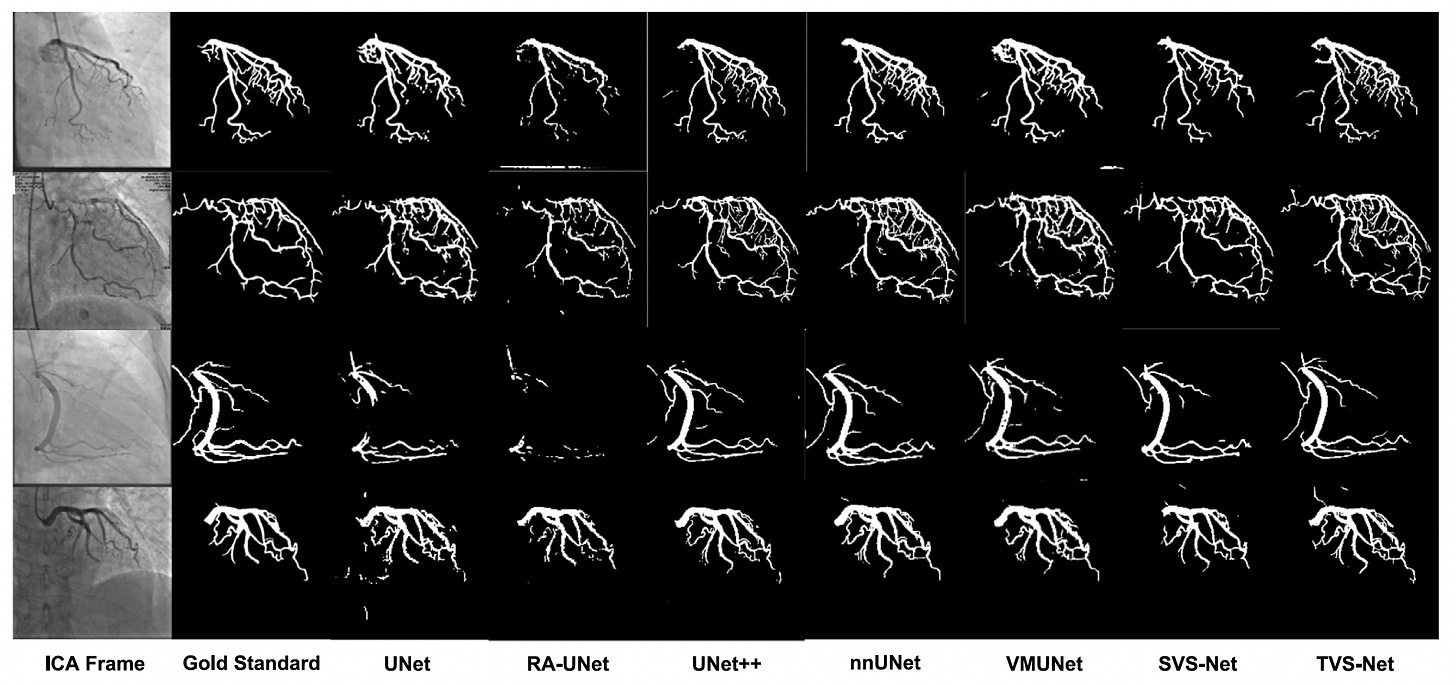
Trained on 323 ICA samples, TVS-Net achieved state-of-the-art performance with an 83.4% Dice score and 84.3% recall, and demonstrated strong generalization on external datasets.
Introduced a densely connected 3D encoder and 2D decoder architecture, enabling robust spatio-temporal feature extraction and precise vessel boundary delineation across multiple ICA frames.
Incorporated a connectivity-preserving energy loss function inspired by elastic energy minimization, effectively maintaining vascular topology and reducing vessel disconnections in the segmentation output.
Outperformed six state-of-the-art models, including nnUNet and SVS-Net, achieving superior results across both public and out-of-distribution datasets without requiring retraining.
Demonstrated the feasibility of weak supervision by surpassing the quality of its own coarse-grained training annotations, even when evaluated against newly created fine-grained gold standard datasets.
TVS-Net establishes a new benchmark for coronary vessel segmentation, offering a scalable and generalizable AI solution for clinical decision support in cardiovascular interventions.
ChemIQ: Testing the Chemical Reasoning Limits of AI
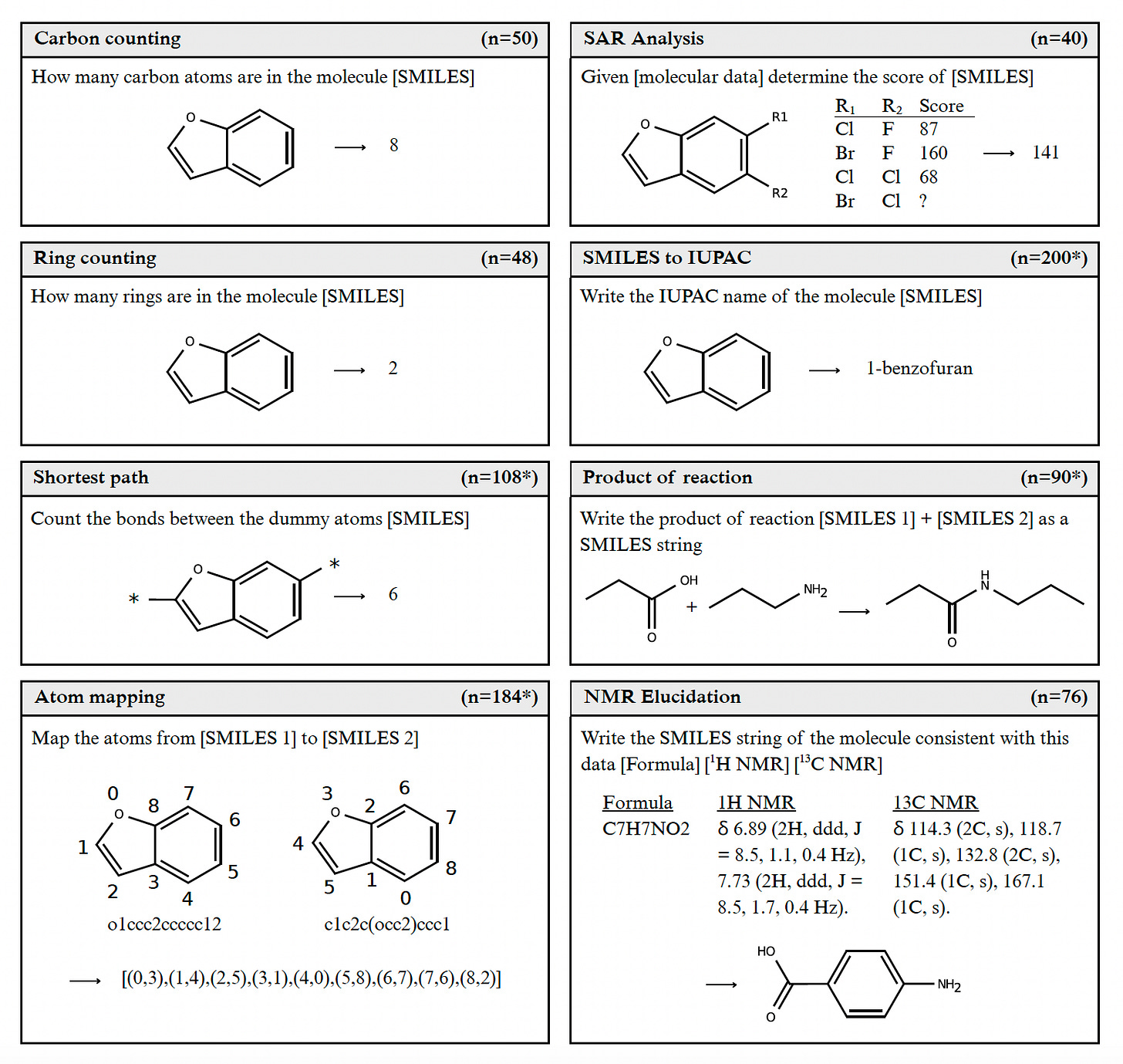
Researchers from the University of Oxford (again yes) introduced ChemIQ, a new benchmark designed to rigorously evaluate the chemical reasoning capabilities of large language models (LLMs). Unlike previous benchmarks focused on multiple-choice questions, ChemIQ requires models to generate short-form answers, better reflecting real-world chemistry tasks.
The benchmark tested models like OpenAI’s o3-mini and GPT-4o across 796 questions involving molecular comprehension and chemical reasoning. The o3-mini reasoning model achieved up to 59% accuracy, while GPT-4o managed only 7%.
Developed a novel set of algorithmically generated chemistry tasks focusing on molecular comprehension, including SMILES to IUPAC conversion, NMR elucidation, and reaction product prediction.
Demonstrated that reasoning-enabled LLMs, particularly o3-mini-high, could correctly name molecules using IUPAC conventions in 29% of cases, a significant improvement over previous near-zero performance.
Showed that o3-mini-high accurately solved 74% of NMR structure elucidation tasks for small molecules, even generating correct SMILES representations for complex molecules containing up to 21 heavy atoms.
Observed that models employing explicit reasoning steps performed significantly better across all tasks, indicating that chain-of-thought prompting and reinforcement learning have a transformative impact on chemical intelligence.
ChemIQ reveals that while reasoning models have made substantial progress, their accuracy remains insufficient for fully autonomous chemistry applications. Still, this marks a pivotal step toward AI-assisted molecular discovery and analysis.
ReactSeq: A Reaction Language Bridging Chemistry and AI
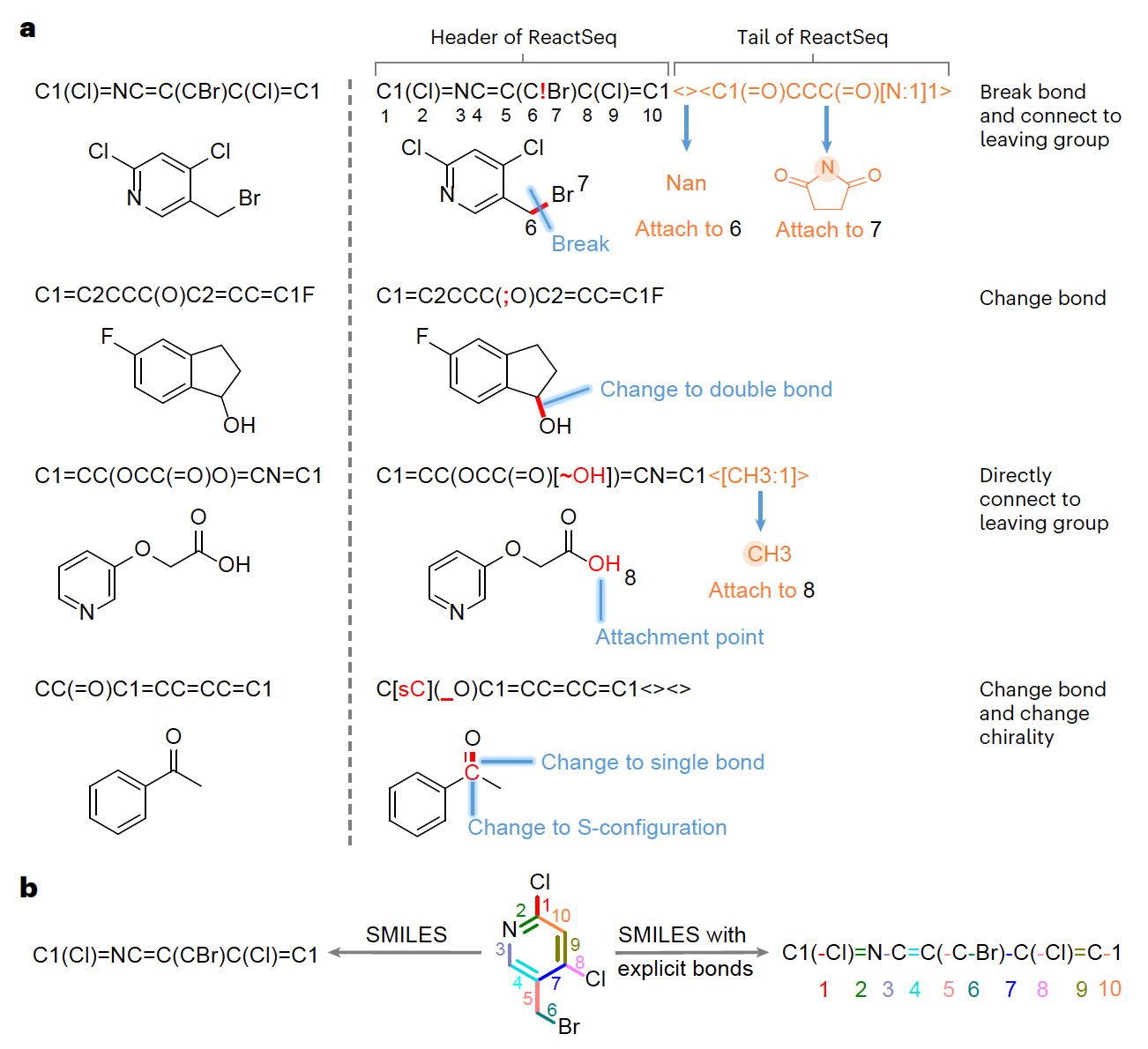
Researchers introduced ReactSeq, a novel reaction description language designed to improve the performance and interpretability of chemical language models. By explicitly modeling atomic and bond changes, ReactSeq enhances retrosynthesis predictions and enables explainable and interactive AI for chemical discovery.
Using ReactSeq, a vanilla transformer outperformed all previous models in retrosynthesis prediction across benchmark datasets without requiring architectural changes.
Defined molecular editing operations (MEOs) such as bond breaking, chirality shifts, and leaving group attachments, allowing precise tracking of chemical transformations at the atomic level.
Enabled human-in-the-loop interaction by incorporating MEO tokens into model prompts, significantly boosting top-one retrosynthesis accuracy from 74.9% to 96.6% when expert guidance was provided.
Produced universal and reliable reaction representations using embeddings of MEO tokens, achieving state-of-the-art performance in reaction classification, similarity retrieval, and yield prediction.
Successfully demonstrated multistep retrosynthesis planning for FDA-approved drugs and new synthetic routes for complex intermediates, highlighting practical applications in drug discovery and development.
ReactSeq provides a critical bridge between symbolic chemistry knowledge and AI, offering a scalable and interpretable framework for advanced chemical reasoning and exploration.
DiffSMol: Shape-Conditioned Diffusion for 3D Drug Discovery
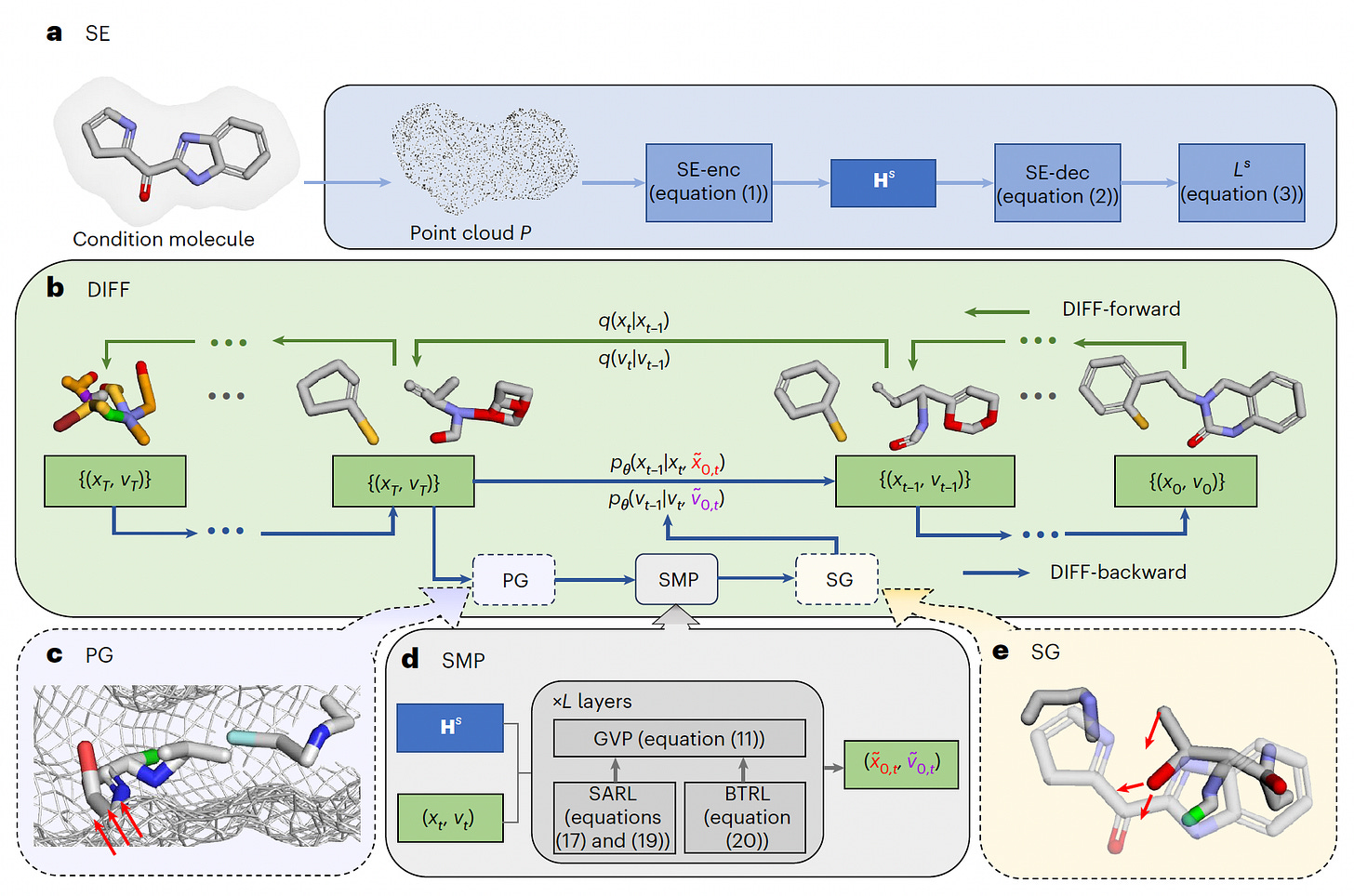
A new study presents DiffSMol, a generative AI framework that designs 3D small binding molecules using ligand shape and protein pocket guidance. By leveraging pretrained shape embeddings and a diffusion-based model, DiffSMol advances beyond traditional ligand- and structure-based drug design methods.
The framework outperforms state-of-the-art approaches in generating novel molecules with high shape similarity, drug-likeness, and binding affinity.
Developed a two-stage pipeline where ligand shapes are first encoded into expressive embeddings, enabling the generation of entirely new molecular structures that retain target binding shapes.
Incorporated iterative shape guidance to boost desirable molecule generation success rates by 448%, reaching 61.4% compared to 11.2% from the best baseline.
Integrated protein pocket guidance to enhance binding affinity predictions, achieving a 17.7% improvement over baselines and generating candidates with favorable ADMET profiles for targets like CDK6 and neprilysin.
Demonstrated a 10x speed advantage over existing methods, producing molecules with better binding affinities, drug-likeness, and synthesizability while significantly reducing computational costs.
DiffSMol establishes a scalable, interpretable AI framework for de novo drug design, offering promising advances for therapeutic discovery and development.
Love Health Intelligence (HINT)? Share it with your friends using this link: Health Intelligence.
Want to contact Health Intelligence (HINT)? Contact us today @ lukeyunmedia@gmail.com!
Thanks for reading, by Luke Yun